Introduction to PGDs
Before thinking about developing a Patient Group Direction (PGD), ensure you are familiar with the information contained in Introduction to PGDs.
About this guide
The preferred way for individuals to receive medicines is for a prescriber to provide care for an individual on a one-to-one basis.
A PGD should not be used where there is an alternative legal mechanism for the administration or supply of the medicine (see Assess the need for a PGD below and When not to use a PGD for further information).
This guide aims to help achieve compliance with legislation and NICE MPG2 Patient Group Directions 2017.
How to use this guide
This guide is designed to help organisations, potential authors and signatories of PGDs consider, plan and follow necessary processes involved in the development and authorisation of PGDs.
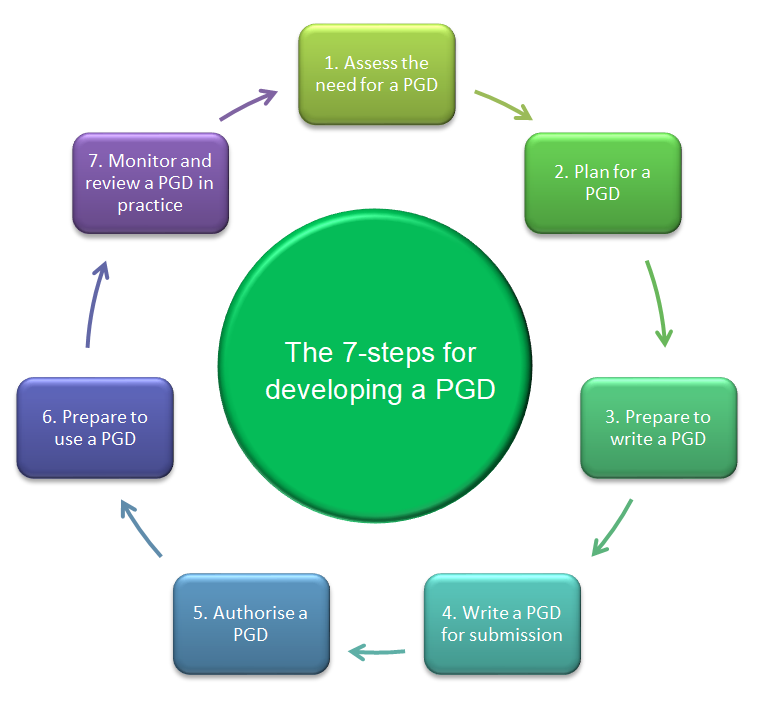
Each stage is shown in the image below and explained in the articles within this series (listed at the top of this article). Do not refer to each stage in this guide in isolation but plan ahead and be familiar with the different stages in the process.
Advice may be repeated throughout the guide because the associated tasks vary at different stages, and this is a stepwise and cyclical process.
Links are added to help navigate the Specialist Pharmacy Service PGD resources for further, more detailed information as well as NICE MPG2 Patient Group Directions 2017.
Key points of developing a PGD
When developing your PGD, key points to note include:
- PGD development requires a multi-disciplinary and sometimes cross-organisational approach
- knowledge about PGD legislation and governance, the service where the PGD is being considered, and clinical expertise is required
- ensure all those involved understand their role and responsibilities
- always consider related processes and policies and plan ahead for implementation
- make sure that there is formal, documented agreement at each stage in the process
The cyclical PGD process
1. Assess the need for a PGD
Whether you’re thinking about creating a new PGD or reviewing an existing one, a PGD may not be the best option. Follow the guidance in “Assess the need for a PGD” below, before going any further.
2. Plan for a PGD
When you know a PGD is the most appropriate option, take time to plan to ensure a structured approach to PGD development. See Planning for a PGD for further information.
3. Prepare to write a PGD
Undertaking preparation steps before writing a PGD can make the process more efficient. See Writing a PGD for more information.
4. Write a PGD for submission
When writing a PGD, ensure you follow local policies, national guidance and involve a range of stakeholders. See Writing a PGD for further information.
5. Authorise a PGD
The process for authorising a PGD may vary locally but there are some key considerations for all PGD authors. See Authorising and preparing to use a PGD.
6. Prepare to use a PGD
Completing some key preparation steps before using a PGD in practice can help to ensure safe and appropriate use of the PGD in practice. See Authorising and preparing to use a PGD.
7. Monitor and review a PGD in practice
Once a PGD is in use, it’s important to ensure it is being used appropriately and safely. See Monitoring and reviewing a PGD in practice.
Before starting
There are some basic points to consider before embarking on developing a PGD.
Preferred mechanisms
The preferred way for patients to receive medicines is for a prescriber to provide care for an individual patient on a one-to-one basis. Consider possible use of Schedule 17 or 223 exemptions, protocols or independent prescribers as alternatives to a PGD. When not to use a PGD contains further information.
Professional standards
Health professionals should refer to their own regulatory or professional bodies standards/guidance where available, including:
Multi-disciplinary working
Do not work in isolation. PGD development requires a multi-disciplinary approach.
Timeframe
A PGD is not a quick fix – it can take several months to consider, develop and authorise a PGD.
Make sure timeframes and deadlines are agreed by all parties involved in the process and realistic, at each stage of the process.
Assess the need for a PGD
Do not use PGDs unless there are clear benefits for care without compromising safety, and there are clear governance arrangements and accountability.
Considerations
Prescribing opportunities
Consider whether there is already an opportunity in the care pathway for the medicine to be prescribed in a safe and timely manner. It may need a review of the systems involved in the provision of medicines to determine if there is, or could be, an opportunity for prescribing.
Utilising the skills of non-medical prescribers (NMPs) or planning for the training of future NMPs may remove the need for PGDs.
PGDs are not intended to replace individual prescribing to save prescribers’ time or to address inefficiencies within systems or pathways.
Other legal mechanisms
Consider whether a PGD is the correct legal mechanism. When not to use a PGD provides further information.
Further information
Update history
- Updated and split into multiple articles
- Additional links to HMR 2012 and relevant SPS pages added
- Broken links corrected
- Broken links corrected
- Link updated
- Published