Scope
This article aims to help you understand when checks add value to medication processes.
Embedding an effective checking practice within a process may minimise inadvertent errors. A check may not always be the most appropriate mechanism to improve safe medicines use.
It is the responsibility of the organisation to assess each step in its own right. This article helps support local decision making.
Further content will be developed to support this topic.
Excluded from scope
Checking whether something is clinically safe, the clinical or validation check, is outside the scope of this article.
Supervision of trainees is outside the scope of this article.
How a check can reduce harm
A check is designed to detect errors, not prevent them.
Within a medicine related task, a check is a process where information is examined and cross referenced to ensure the outcome aligns with the instruction or documentation, and vice versa. For example, the medicine name matches the documentation.
The aim is to reduce the risk of an error leaving the service or reaching the patient, and to provide the necessary level of assurance of the quality and safety of the process.
Definitions
Checks can be used across all processes in the journey of a medicine, from procurement to administration.
Self (single) check
For the purposes of this article, a self-check is when the person who has completed the task checks and confirms the accuracy of their actions before moving onto the next stage.
All individuals are responsible for ensuring they complete a self-check before processing to the next stage, even if the next stage in the process is an independent second check by a different person.
Independent second check
An independent second check (also called a double or second check) is when two people undertake the checking process independently of each other before moving on to the next stage in the process. Both people will separately verify that the information being examined aligns with the documentation or instruction, without knowing the results of their colleague.
To be effective, both checks must be completed independently to prevent those checking being influenced or led by each other.
Value and limitations
Introducing an independent second check to a process may not be the most effective way to reduce harm or prevent errors occurring. There may be other system focussed interventions in the design of the process which are more effective and make the error easier to identify, or less likely.
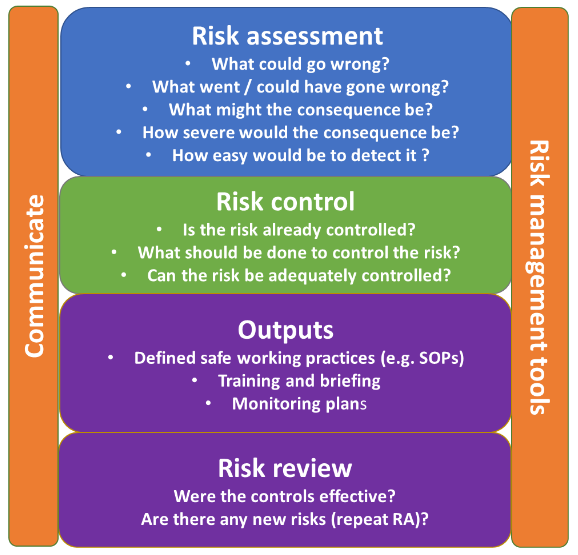
Risk assessment
Undertaking a risk assessment (RA) can be a useful tool to assess and review the value of an independent second check during a medication process.
The process should be mapped, and the risk of each stage in the process assessed. Organisations may have their own risk management policy to support this. See an example of a risk assessment model below.
Any risks, and actions to mitigate them, must be clearly documented. This may require more than one action.
Risk assessment model
Based on ICH Q9 guidelines – Quality risk management.
Value of an independent second check
Including an effective independent second check within a medicine related process may increase opportunities for detecting errors and reduce the risk of harm.
However, it is not practical or feasible to include an independent second check for every step of a process. Situations in which an independent second check may be of more value could include (but not limited to):
- complex or high-risk medicines
- complex of high-risk situations
- high risk route of administration
- processes completed by new of inexperienced staff
- calculations, even when the calculation is very simple
- any step where a member of staff feels that a check would be beneficial.
Limitations of an independent second check
An independent second check can also have limitations and can bring its own risk if not introduced correctly. Examples of this include:
- practical problems such as extra resource or disruptions in workflow required to carry out checks
- independent second checks not being possible in some scenarios, for example lone workers or remote locations, in which case the practitioner could perform their own independent second check by separating the two checks
- an environment with distractions and interruptions
- false sense of security and presuming someone else will catch the mistake
- variability and inconsistency in how checks are performed
- it being seen as a quick fix for problems in systems or process design.
Implementing an independent second check
When implementing a new safety intervention such as an independent second check, the conditions or limitations on its use should be clearly communicated to staff so they are aware of their responsibilities. It is also necessary to monitor the use of the check in line with legislative requirements and organisational policy.
Local standard operating procedures and or policies should outline specific details on responsibilities, training, systems of work and processes.
An organisation may choose to increase or decrease the checking requirements for each process or staff group dependent on location-specific factors such as (but not limited to):
- experience level and competence of staff
- types of process being undertaken
- new process or medicine in use
- location of process being undertaken, especially if outside of usual environment.
It is important to note that these recommendations may additionally need to be adapted to suit the abilities of individual practitioners.
The organisation will need to describe which staff groups can be included in the checking process, and the responsibility and accountability of the practitioner. Staff to consider include registered and non-registered individuals, and may include the patient. Competence must be assured.
Governance
A formal governance system should clearly define the responsibilities for all staff undertaking processes for all stages of the medicines journey, from procurement to administration. It is recommended that there is an overarching local policy, which includes reference to the key fundamentals.
The organisation’s policy for the use of independent second checks (and other safety interventions) should specify suitable audits and timetables, along with the reporting mechanism and the responsible committee.
Monitoring
Organisations should have processes in place to monitor, review and re-evaluate the implementation of safety interventions, which may include, but are not limited to:
- audits of compliance with the agreed process
- audits of record keeping and documentation
- monitoring of adverse incidents.
Update history
- Risk assessment model added and minor formatting changes
- Published